Licensed Radiation Labs for Development and Production of Clinical Trial Charges
We offer fully licensed radioisotope labs, stable supply arrangements for almost all pharmaceutical radioisotopes, a state-of-the-art infrastructure including logistics, and a highly experienced staff that can be seconded to help with your preclinical and clinical projects.


Key Features
Background
For more than 25 years, Eckert & Ziegler has been the partner of choice for pharmaceutical companies that develop radiotherapeutics, radiodiagnostics, or radioactive medical devices. Our world-wide network of GMP suites in Europe, Asia, and the Americas will derisk and speed-up the pre-clinical or clinical development of your radiopharmaceutical. Our customizable clean rooms are equipped with hot cells for alpha, beta, and gamma emitters, the radiosynthesis, quality control and further equipment and are operated in accordance with (c)GMP conditions.
Service Portfolio
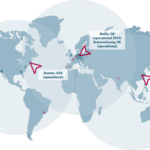
Locations
Whether you are seeking services for the European, North American, Asian, or even the global market, we have you covered. With locations in Germany (Berlin and Braunschweig), the USA (Wilmington, MA) and China (Jintan, operational ready 2025), we can meet your global needs for a development and manufacturing partner.
Capabilities
All sites have optimal accessibility to global transportation hubs to facilitate raw material supply chains and product shipment. Our proximity to biotechnology clusters and the scalability of laboratory spaces assures the best possible infrastructure to our customers.
Touring our GMP Suite in Berlin, Germany
Join us on a tour of our new state-of-the-art GMP Suite at our headquarters in Berlin, Germany. Take a look at the cutting-edge facility designed for contract manufacturing of your investigational radiopharmaceuticals.
Product specific questions?
Eckert & Ziegler
Radiopharma GmbH
Robert-Rössle-Straße 10
13125 Berlin
Germany
Eckert & Ziegler
Radiopharma, Inc.
25 Upton Drive
Wilmington, MA 01887
USA